Some of our trusted customers








Julius Breuch
Managing Director
Gerd Breuch
Management Consultant
Eugen Reger
Head of QM/RA
Matthias Schön
Managing Director
Andreas Happold
Business Development Director
Sina Bieler
Project Manager
Christian Lange
Head of System Development
Susi Pfullmann
Head of V&V
Mohamed Choukri
Chief Engineer
Rabea Gabel
Test Engineer
Kathrin Kehrle
Head of Innovation and Education
Carina Brieskorn
Risk ManagerAbout Us
Headquartered in Hamburg, D.Med Consulting GmbH (“DMC”) is one of the leading specialists in medical device technologies. From the initial idea to the finished product, we develop new tailored solutions in cooperation with our clients, re-engineer existing medical devices, and prepare your market launch by making use of our great network of partners from different areas.
DMC was founded in 2011. Since 2019 it is a joint venture of Fresenius Medical Care, the global market leader in the field of renal care and dialysis, and D.med Healthcare Group, a global provider of medical services and medical products in nephrology, diabetes, and other fields of internal medicine. We bring together two complementary pillars to provide our clients with a full range of services from concept to implementation.

About Us
Headquartered in Hamburg, D.Med Consulting GmbH (“DMC”) is one of the leading specialists in medical device technologies. From the initial idea to the finished product, we develop new tailored solutions in cooperation with our clients, re-engineer existing medical devices, and prepare your market launch by making use of our great network of partners from different areas.
DMC was founded in 2011. Since 2019 it is a joint venture of Fresenius Medical Care, the global market leader in the field of renal care and dialysis, and D.med Healthcare Group, a global provider of medical services and medical products in nephrology, diabetes, and other fields of internal medicine. We bring together two complementary pillars to provide our clients with a full range of services from concept to implementation.
Our services
Our key experience areas
Cost reduction of medical devices
Technical assessments & Proof of concept
End-to-end development of your medical device
Do you need support to develop your medical device? Leverage our extensive end-to-end development expertise, validated through numerous successful collaborations with industry leaders in the medical device field. From concept to commercialization, we provide comprehensive support throughout the entire development lifecycle.

Cost reduction of medical devices
Technical assessments & Proof of concept

Technical assessments & Proof of concept
End-to-end development of your medical device
Success Stories



Fresenius 5008S CorDiax and 6008 CAREsystem

Ex Vivo Lung Perfusion (EVLP)

NephroFlow

Regulatory Affairs Consulting
- Conducting a meticulous Gap Assessment to identify the new product requirements.
- Thoroughly updating the technical file to align with the MDR requirements.
- Efficiently managing the implementation of normative mandated tests, including functional safety (IEC60601-2-16), electrical safety (IEC60601-1), and biocompatibility (ISO10993).
- Skillfully creating a clinical evaluation in accordance with MEDDEV 2.7.1 Rev 4.
- Leading the communication with the notified body to ensure seamless compliance.
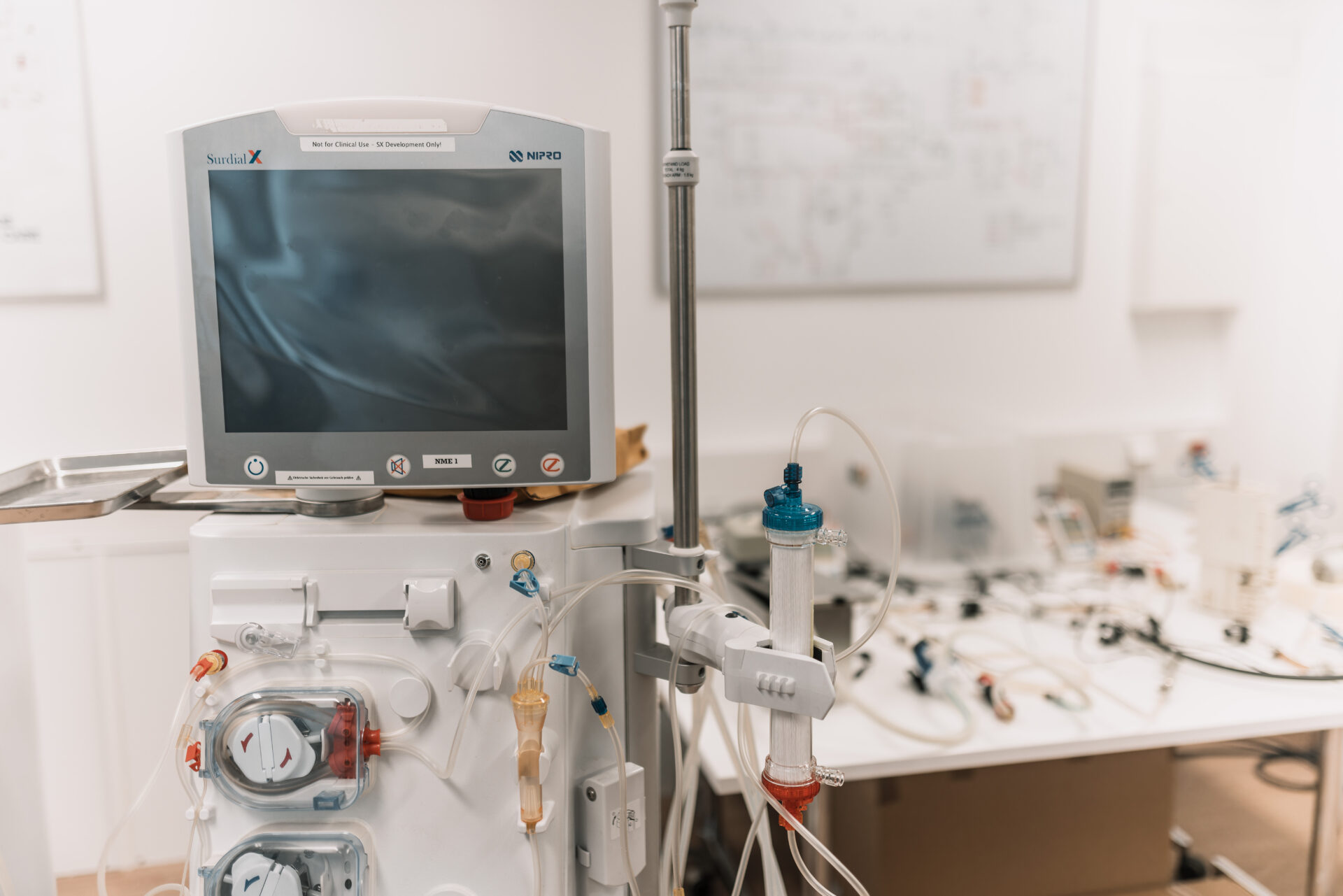
Nipro Surdial X Haemodialysis Machine

Monitoring & Database Software for Dialysis Clinics
Quality & Regulations
